Products
Pharmaceutical metal detector for tablets and capsules
INQUIRYPharmaceutical metal detector for tablets and capsules

Anritsu pharmaceutical metal detector with three leading technologies offers the highest and most stable product quality control on the market.
|
Ensured on the production line High stability against external factors
⇓ |
Industry-leading level High sensitivity
⇓ |
Maintenance of high production quality Verification
⇓ |
| Achieve high level of quality | ||
.
High stability metal detector - ensures efficient detection of metals/ contaminants
The main causes of lower stability in metal detection are vibration, static electricity and electrical noise from peripheral devices that destabilize the magnetic fields in the detection heads. Anritsu pharmaceutical metal detector with improved resistance to these negative factors ensures stable and accurate detection of contaminants.
| Vibrations from the upstream and downstream line equipment | ||
| Static electricity of tablets and capsules | 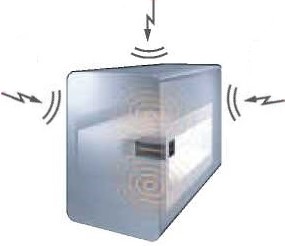 |
Inverter noise from the upstream and downstream line equipment |
The metal detector is vibration-resistant
Vibrations from the upstream and downstream line equipment, such as tablet presses, capsule filling machines, and powder removers, can cause false rejections. Digital signal processing is integrated to minimize vibration noise by improving stability against vibration.
Reduction of static electricity
Static electricity of tablets and capsules can cause false rejections. An antistatic chute (optional part) reduces amplification of static electricity and prevents malfunctions.
Resistance to noise from peripheral devices
Inverter noise from upstream and downstream line equipment can reduce stability of the metal detector. Improved signal processing increases resistance to inverter noise generated by the upstream and downstream line equipment, ensuring stable detection sensitivity on the production line. The metal detector is equipped with a unique function that eliminates the impact of the generated noise on the metal detector.
Detection sensitivity – industry-leading level 
Processing of metal detector’s signal for the inspection of pharmaceutical products significantly reduces the effect of the product on the detection sensitivity, providing the highest detection sensitivity on the market. Metal detector’s detection sensitivity: Fe (ferrous metal) 0.25 mm, Non-Fe (non-ferrous metal) 0.30 mm, SS 316 (stainless steel) 0.40 mm. There is no need to set samples before testing for most tablets and capsules. No complicated settings are required to achieve high detection sensitivity. Even tablets and capsules that contain difficult-to-detect contaminants, such as iron, can be adjusted to optimal sensitivity by feeding the product only once.
Analyse magnetic and non-magnetic materials in metal contaminants in an instant
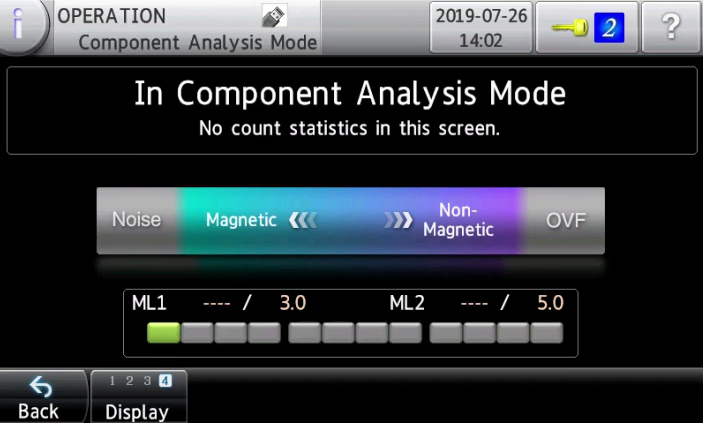
Anritsu pharmaceutical metal detector can detect whether a contaminant is a magnetic or non-magnetic material without damaging the tablets and capsules. In the common method, a special inspection device is used to perform the endurance test and to identify the contaminants. However, it takes time to obtain results using such method. The new function helps the operator to check contaminants' properties before performing the endurance test.
Verification ensures a high level of quality control
The built-in monitoring function verifies correct operation of the metal detector.
Continuous internal monitoring of the metal detector
The built-in automatic monitoring function constantly controls the internal equipment during the production and instantly reports a fault to alert the operator of a problem occurred.
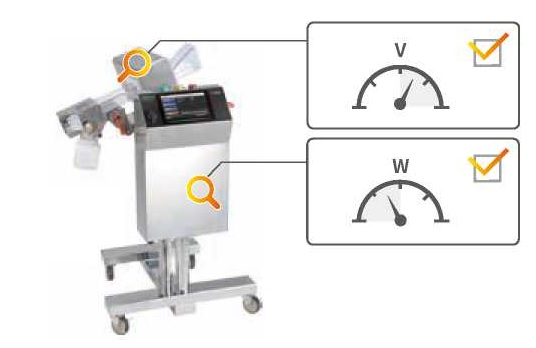
Self-diagnosis of detection activities (patented)
This diagnostic feature allows the operator to verify whether the machine maintains the same level of performance, as originally set in the factory.
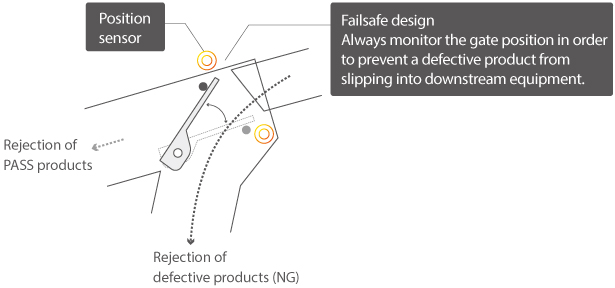
Duplex monitoring system – in case of product rejection
The rejection unit is equipped with a position sensor on both the PASS and NG side to check the position during the start-up of the machine and at the time of contaminants’ detection. Safe design allows the rejection unit to stay in the NG direction at a time when electricity is not conducted and abnormalities occur, preventing passing of defective product, as well as any product that does not meet the evaluation criteria of PASS direction.
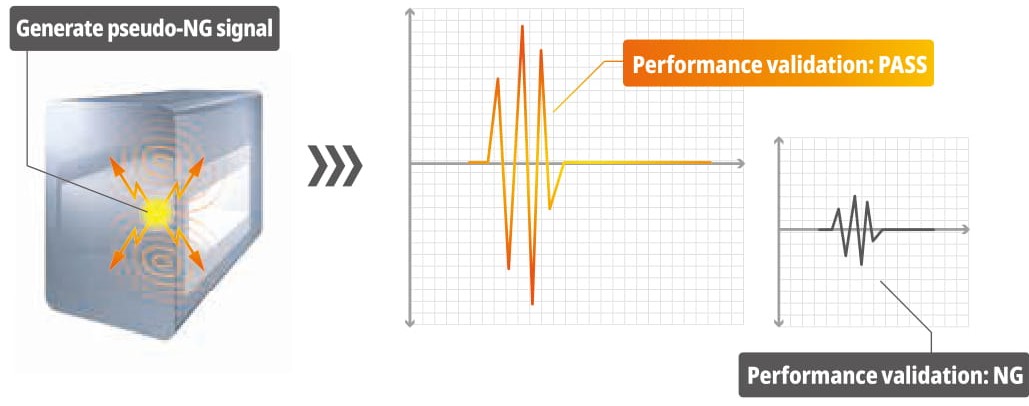
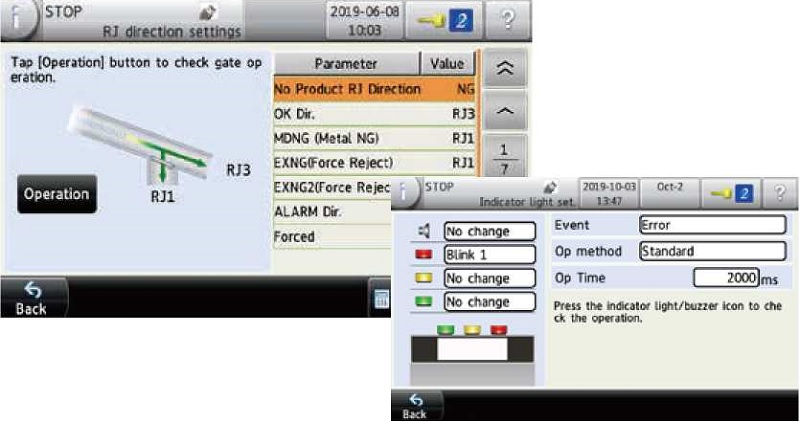
Support function for validation process such as IQ/OQ
Support functions allow the operator to confirm the operation check of the rejection gate and the correct setting of the sensors on the screen to ensure the IQ (installation qualification) and OQ (operation qualification) processes. With these functions, the operator can output the necessary information to create a document with verification results.
Compliance with FDA 21 CFR Part 11 ![]()
In addition to high-precision inspection, it is important for pharmaceutical metal detectors to manage and record production and inspection data. Anritsu pharmaceutical metal detector complies with the provisions of Section 21 Part 11 of the U.S. Food and Drug Administration (FDA) Act regarding user authentication, audit trails, and data encryption/decryption.
|
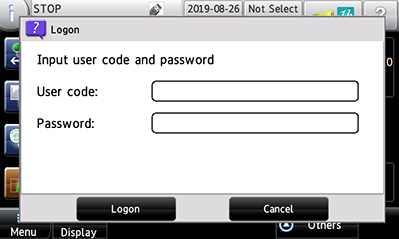
Authentication (user management) Authentication with a username and password is required to turn on the metal detector. To prevent unauthorized activity, access level can be set for each user individually. |
 |
|
Audit trails The system internally records the history of production-related activities and measures, as well as the results of the performance testing. This data can be used to prevent fraudulent or incorrect activities and to analyse the causes of such activities. |
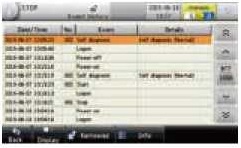 |
|
Data encryption and decryption Various data can be easily transferred, including audit trail statistics and parameters. |
|
|
The Smart Guide allows the operator to follow the correct operating procedure, to ensure that the standard operating procedures (SOPs) are followed correctly. |
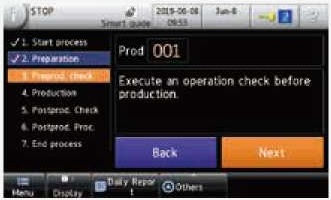 |
Validation support
Anritsu also provides IQ/OQ checklists and on-site support during the on-site performance qualification (PQ) process.
Computer System Validation (CSV) supporting guidelines:
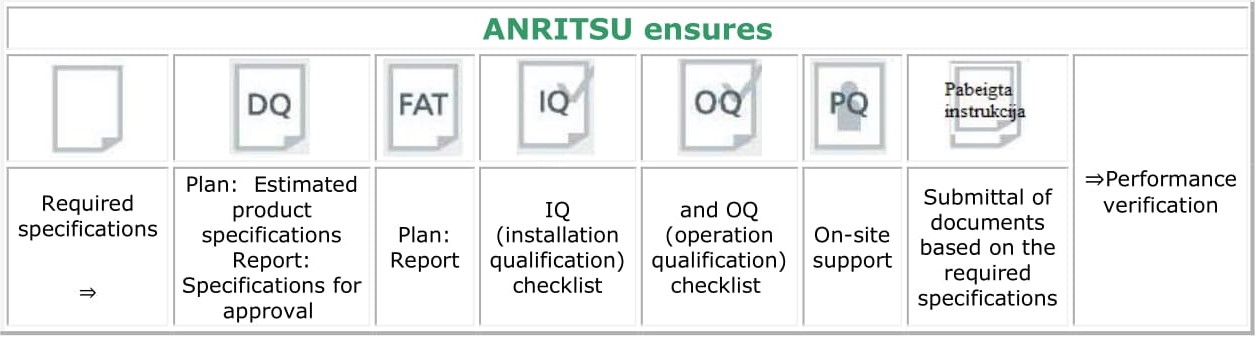
.jpg) |
Support for proper use of data in accordance with FDA CFR 21 Part 11 functions. Ensurance of data integrity, as provided for in the FDA CFR 21 Part 11, using the data obtained from the network-connected machine. ° Eligibility authentication (user management) ° Audit trail ° Production process analysis ° Data quality |

.
Tool-free removal of parts
|
Parts that are in direct contact with pharmaceutical products, such as the feeding chute, rejection box and defective product box, can be easily removed and attached without the use of tools. |
 |
Simple adjustment
The angle of the chute and swing angle can be adjusted effortlessly without the use of tools.
l








